Bio/Pharmaceutical products encounter a wide range of polymeric materials on their journey from the production line to the patient, with plastic and rubber surfaces present at almost every stage of a product’s lifecycle. While these materials are extensively used in the manufacture and storage of medicines, the leaching of these potentially hazardous substances into the products themselves can pose a risk to human health.Extractable & Leachable studies enable drug and device manufacturers to identify and quantify the risks of potentially toxic impurities migrating into a drug product from container closure systems, processing equipment or medical devices. In this article, we take a closer look at Extractable and Leachable species and the design of E&L studies as well as test methods which are deployed for accurate determination of E&L contamination.

Extractables: Organic and inorganic chemical species that can be released from the surfaces of components used in the manufacture and storage of drug products under laboratory conditions (accelerated or exaggerated temperatures, solvents or surface exposure).
Leachables: Organic and inorganic chemical species that can be released from the surfaces of components used in the manufacture and storage of drug products under conditions of normal use.
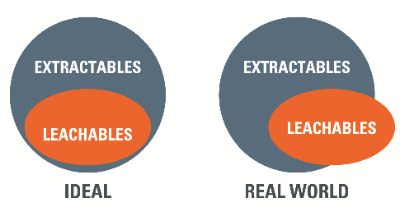
Extractables represent the worst-case scenario regarding release of mobile chemical species from manufacturing and packaging components during forced extraction. Generally, leachables should then comprise a sub set within this pool of mobile chemical species, released under the gentler conditions of on-shelf storage or use.
Rigorous testing workflows must be employed to make sure extractable and leachable compounds are fully understood and do not impact patient safety or pharmacological activity. The need to detect a wide variety of compounds necessitates the application of a range of different analytical techniques and testing protocols, making E&L studies often lengthy and complex.
In practice, however, the leachables study may identify additional species that were not observed during the preceding extractables study. Thus, the set of leachable species is not wholly included within the set of extractables, but there is strong overlap between both sets (figure 1).
There are multiple sources which can contain leachable species, and some common examples are detailed below:
Leachable species to consider as part of an E&L study include:
The extensive array of materials employed in the production and preservation of therapeutic goods, along with the considerable range of leachables that could potentially migrate into the final product, necessitates the deployment of structured and thorough E&L studies.
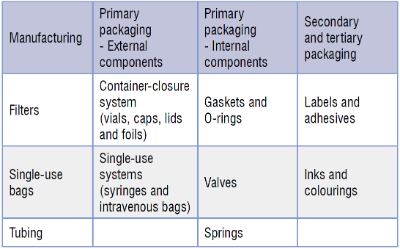
Leachables that migrate into pharmaceutical products from manufacturing and packaging systems require identification and monitoring over the shelf-life of the product. The collated data permits a toxicological assessment to be made with respect to any extractables and leachables found, ensuring patient safety.
One must also determine the Safety Concern Threshold (SCT) for the product under investigation.
The concept of Safety Concern Thresholds (SCTs) was first introduced by the Leachables and Extractables Working Group of the Product Quality Research Institute (PQRI). SCTs are defined as the threshold dose at which an individual leachable would not pose a safety concern, including potential effects such as carcinogenicity.
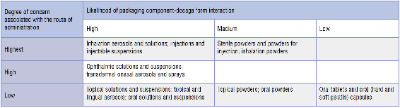
Two factors which have a significant impact on the SCT are the route of administration and the degree of product-packaging contact. To assign risk from leachable species to the pharmaceutical product under investigation, the USFDA developed the following matrix:
Products such as aerosols and injectables have the highest associated risk, whilst oral tablets and capsules have the lowest risk.
The PQRI have recommended that the high risk SCT is set at 0.15 μg/day, whilst the low risk SCT is set at 1.5 μg/day, both having been justified from toxicological and safety perspectives.
Under certain conditions, such as short-term exposure or in the treatment of a life threatening condition, the SCT can be raised above 1.5 μg/day.
An E&L study comprises two interlinked yet distinct projects. The extractables study aims to identify any substances originating from manufacturing components (wherever relevant) and the packaging system that could potentially migrate into the pharmaceutical product during storage or use under typical conditions. This establishes a foundational assessment for any subsequent leachables study, which includes a series of tests carried out at predefined time-points on the pharmaceutical product for the duration of its shelf-life.
The manufacturing, delivery and storage components under investigation are often extracted in isolation of the pharmaceutical product. Key points to consider are the number of components and material types that are to be tested, and the solvents with which to perform the extractions.
Simple storage systems, e.g. glass ampoules or plastic bottles with screw caps, will have a limited number of components. However, more intricate units, e.g. pump dispensers containing O-rings and springs, will contain multiple components which all require investigation. Secondary and tertiary packaging also needs to be considered at this stage.
To ensure a comprehensive representation of both organic and inorganic extractable species, it is imperative to perform extractions utilising a diverse range of solvents possessing distinct solvating capacities. In the context of liquid formulations, it is advisable to opt for solvents that closely mimic the pharmaceutical product's final composition, thereby facilitating the generation of an extractables profile that reflects a worst-case scenario. However, caution must be exercised to prevent the utilisation of solvents with excessive potency, as their aggressive nature may induce material degradation and consequently lead to an artificially inflated array of extractable species.
Two or three solvents are typically chosen, but more can be used if considered appropriate.
Common examples include:
The material type under investigation is an important consideration. It is necessary to perform extractions using all selected solvents on plastics and rubbers. However, conducting organic solvent extraction on metal springs would primarily yield inorganic impurities, resulting in limited usefulness. Therefore, optimising the extraction process requires thoughtful assessment of solvent compatibility with the specific components under investigation.
In an E&L study, predefined time-points are established prior to initiation, which can be conducted in parallel with a stability trial. Samples obtained at these timepoints undergo targeted screening with validated methods for leachables previously identified from the extractables study and untargeted screening for any newly discovered species during the leachables study. If any new leachables are found to exceed the Safety Concern Threshold (SCT), they are identified and subjected to toxicity assessment.
Crucial to a successful study is the creation and storage of appropriate controls and samples. The controls should be stored in such a fashion that there is minimal risk of leachable ingress, and carefully labelled avoiding the use of inks and adhesives directly on the container. Leachable samples may be stored inverted as well as upright (e.g. bottles fitted with caps or lids), and specific storage conditions (e.g. 4°C, 25°C/60% RH, 40°C/75% RH) should be carefully considered.
Analysing E&L samples
In general, extractables and leachables can be divided into three broad groups:
Well established analytical methods are required to analyse all samples and can be used across both studies. Figure 2 exemplifies the typical analytical strategy employed.
Liquid chromatography–mass spectrometry (LC-MS) analysis permits the analysis of larger, non-volatile species. Direct injection gas chromatography mass spectrometry (GC-MS) analysis permits analysis of both volatile and semivolatile species. Head-space GC-MS analysis is an option when significant volatile species are expected. Elemental impurities are analysed and quantified by inductively coupled plasma mass spectrometry (ICPMS).
Well established analytical methodologies are a critical prerequisite prior to initiating research, although they can be customised to accommodate the diverse array of pharmaceutical products. For leachable studies, feasibility is a necessary prerequisite for validation of the method to ensure that the pharmaceutical product under investigation does not exhibit inhibitory matrix effects allowing successful recovery of extractables from the sample matrix, thus avoiding the necessity for method redevelopment and subsequent revalidation.

The complex nature of the E&L studies requires thorough planning, access to a variety of complex analytical hardware and expertise running validated methods. The investigative nature of the work demands a team of analysts with capabilities across method development & validation, molecular identification and toxicological evaluation, to ensure that the study is run in a smooth and efficient manner and data is interpreted accurately.
With thorough planning, appropriate analytical hardware and in-depth knowledge in place from the beginning, an E&L study can be run efficiently and successfully, ultimately ensuring that patient safety is maintained.
SGS Health Science enables the medical and health innovators of the world to deliver life-changing solutions in the fastest, safest and most efficient way, helping improve the lives of many. We provide the highest quality services, reliable expertise and guidance through our network of laboratories located around the globe.
SGS has a global expert network with more than 20 years of experience in E&L studies:
The SGS India Centre of Excellence in Navi Mumbai is a USFDA approved facility and can undertake complex E&L studies, which are an important part of drug product development and materials qualification.
SGS capabilities for E&L studies include:
This state-of-the-art facility is equipped with high-end instruments including HRMS, LCMS/MS, GCMS/MS, ICPMS which are extensively used for conducting extractable and leachable analyses.
The extractables and leachables testing and assessment strategy, followed by SGS, includes:
The SGS laboratory is equipped to conduct comprehensive study design and testing for extractables and leachables in finished pharmaceutical packaging, process equipment, and medical devices. Additionally, it undertakes analysis for leachables in final products. These services can be customised based on specific requirements or aligned with the guidelines provided by the Product Quality Research Institute (PQRI).
Our experts can be reached at: in.pharmaqc@sgs.com
SGS is world’s leading Testing, Inspection and Certification company. We are recognised as the global benchmark for sustainability, quality and integrity. Our 97,000 employees operate a network of 2,650 offices and laboratories, working together to enable a better, safer and more interconnected world.
Wherever you are, whatever your industry, our experts worldwide provide specialised solutions to make your business faster, simpler and more efficient.