
Nitrosamine describes a class of compounds with a nitroso group's chemical structure bonded to an amine. They are common in water and foods, including cured and grilled meats, dairy products, and vegetables. Everyone is exposed to some level of nitrosamines. However, the Medicine Regulatory Authorities first became aware of the presence of the nitrosamine impurity, N-nitrosodimethylamine (NDMA), in products containing valsartan in July 2018. Subsequently, nitrosamine impurities were detected in other medicines belonging to Sartan Family. In September 2019, it was detected in antacids such as Ranitidine and Nizatidine, and in May 2020, detected in metformin extended-release formulation, etc1,2,3. Nitrosamine impurity has led to more than 1500 voluntary recalls of several anti-diabetic (metformin, pioglitazone), hypertensive (valsartan, losartan), and histamine blocker (ranitidine, nizatidine) drugs and their combination.
Nitrosamine impurities constitute a significant concern because of their impact at the genetic level: (Figure 1)
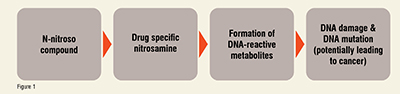
Owing to this, the regulators demanded detailed risk assessment and clear communication from the drug manufacturers on the presence of nitrosamine impurities in the application with the agency. So that the quality, efficacy of the drug, and patient safety are not compromised.
The risk assessment should not be limited to drug substances/API's but also requires evaluating starting materials, solvents, reagents, and catalysts. Awareness of the supply chain of raw materials became an essential factor in assessing and controlling the impurities levels. The drug manufacturer and API producers may not be aware of nitrosamine contamination in raw or starting materials they have sourced from vendors. As illustrated in the diagram, sources of nitrosamines could be through a.) Primary source – drug substances b.) Secondary source – excipients, solvents, packaging, etc. c.) Formation through a mechanism other than drug substance degradation. (Figure 2)
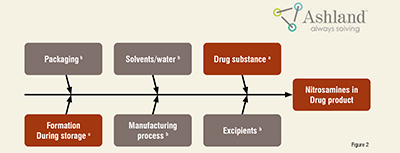
The nitrosamines impurities can also form during the manufacturing process of drug substances/products or the shelf-life storage period of the final drug substances/products.
Based on the above, the drug manufacturer/formulator is responsible for a comprehensive risk assessment for their final drug product and reports to regulatory authorities. The drug manufacturer should consider and apply the below 3-way approach in the final formulation risk assessment. (Figure 3)

Step 1: Assessment: Risk mitigation of all the input sources of the drug formulation and appropriate action.
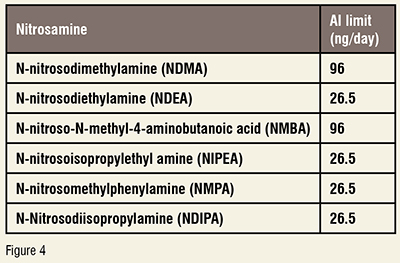
Step 2: Testing:Below is the FDA's recommendation for acceptable intake (AI) limits for the nitrosamine impurities in drug products:(Figure 4)
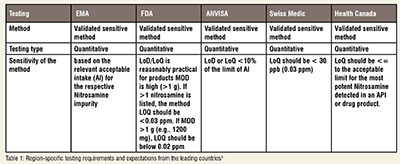
Most methods used are Quantitative on LC-MS/MS method using APCI in positive MDDe and GC-MS /MS with Liquid extraction for Nitrosamine.
As stated in the IPEC federation position paper published in March 2022, Excipient manufacturers are diverse. As a proactive approach, excipient manufacturers could include the relevant and available information on nitrosamine impurities for an excipient in the form of a declaration to customers to mitigate the risk in drug product development. However, the responsibility for overall risk assessment for the presence of nitrosamines in a drug product lies with the MAH or the drug product manufacturer, depending on the region4.

Excipient manufacturers can support drug manufacturers with the following:
• Understanding the manufacturing process and the basic chemistry of the raw materials used. This potentially rules out the likelihood of nitrosamines.
• IPEC questionnaires that identify checkpoints, namely the manufacturing process, vulnerable amines, and nitrosating agents to be assessed.
Furthermore, excipients are also discussed a lot for their nitrites - common precursors for nitrosating agents- reported in many excipients at ppm levels. There is a lot of information on nitrite data in excipients in the public domain.
Therefore, as an excipient manufacturer, Ashland continuously monitors and controls the Nitrite levels in all our excipients and ensures the consistency of nitrite levels in all the lots. We have tested all excipients products for simple nitrosamines, such as N-Nitrosodimethyl amine (NDMA), N-Nitrosodiethylamine (NDEA), N-Nitroso-N-dipropylamine (NDPA), and N-Nitroso-N-butylamine (NDBA), the same are not present in our experiments.
Metformin HCl ER Tablets are one of the most recalled formulations from many pharmaceutical manufacturers because of nitrosamine issues.
In view of the same, Ashland tested lots of low Nitrite Benecel K100M XRF in Metformin HCl ER Tablets. All tablet formulations with low Nitrite Benecel K100M XRF showed unchanged NDMA levels even at 40°C/75%RH for 3 months.
Typical values of nitrites were tested using Ashland's in-house "post-column derivatisation HPLC with UV detection method with a detection limit of 6 ppb. Nitrates were tested using in-house "Ion exchange HPLC with UV detection method" with a detection limit of 100 ppb. NDMA was tested using the "GC/MS-SIM method" with a detection limit of 5 ppb.
Ashland is committed to supporting our customers on upcoming regulatory challenges and technical queries by providing product-related risk assessments and technical discussions.
Abbreviation:
1. DNA - Deoxyribonucleic acid
2. API - Active pharmaceutical ingredient
3. FDA - Food and Drug Administration
4. LC-MS/MS - Liquid chromatography-mass spectrometry
5. APCI -Atmospheric pressure chemical ionization
6. GC-MS /MS - Gas chromatography-mass spectrometry
7. LoD – Limit of Detection
8. LoQ – Limit of Quantitation
9. MDD - Minimum Daily Dose
10. CAPA - Corrective and preventive action
11. NA - Not Available
12. GMP - Good manufacturing practice
13. ANVISA - Brazilian Health Regulatory Agency
14. EMA - European Medicines Agency
15. IPEC - International Pharmaceutical Excipients Council
16. RH - Relative humidity
17. HPLC - High-performance liquid chromatography
18. UV - Ultra Violet
19. GC/MS-SIM - Gas Chromatography-Mass Spectrometry - Selected ion monitoring
References:
1. https://www.who.int/news/item/20-11-2019-information-note-nitrosamine-impurities
2. https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m7r1-assessment-control-dna-reactive-mutagenic-impurities-pharmaceuticals-limit_en.pdf
3. https://www.fda.gov/media/141720/download Control of Nitrosamine Impurities in Human Drugs - Guidance for Industry
4. https://www.ipec-europe.org/uploads/publications/202203-if-nitrosamines-position-paper-final-1646123452.pdf